Our research focuses extensively on the underlying molecular mechanisms of sleep and cognitive dysfunction. Poor sleep quality is associated with neuropsychiatric illness and impaired brain function. We also evaluate new therapeutic approaches to alleviate these outcomes.
Role of kynurenic acid (KYNA) in cognition
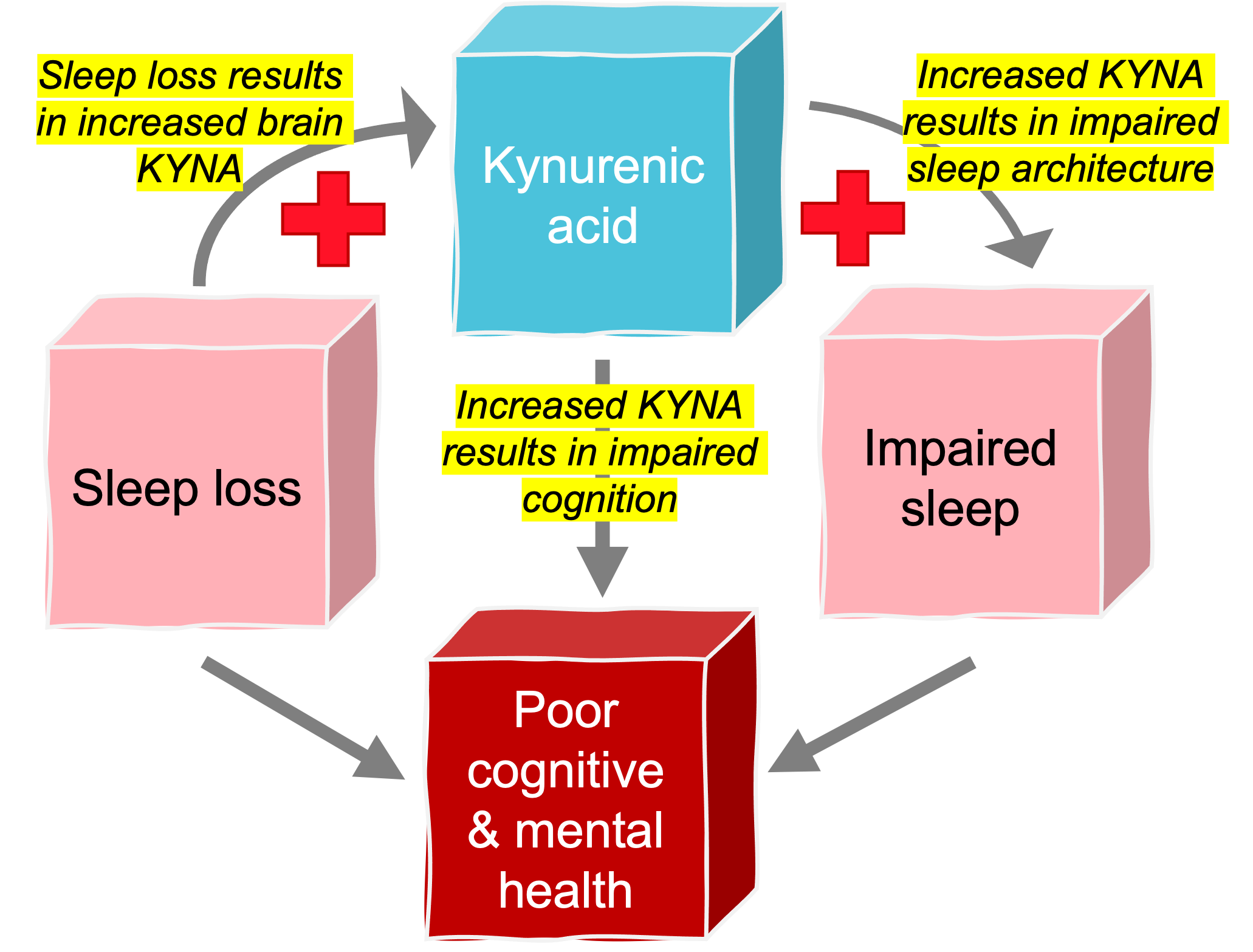
Metabolites of the kynurenine pathway of tryptophan degradation, including kynurenic acid (KYNA) and quinolinic acid, are increasingly understood to play major roles in CNS development (reviewed in Notarangelo and Pocivavsek, 2017), function as neuromodulators in adulthood (reviewed in Pocivavsek et al. 2016), and participate causally in pathological states (reviewed in Campbell et al. 2015). KYNA acts as an endogenous antagonist at glutamatergic and cholinergic receptors, which are richly endowed in the hippocampus and implicated in mediating cognitive function. KYNA has also been extensively implicated in the pathology of psychiatric disorders, including schizophrenia, where studies using cerebrospinal fluid (CSF) or post-mortem brain tissue of patients with schizophrenia suggest that an excess of KYNA might play a causative role in the disease. Targeted inhibition of KYNA formation is being investigated as a therapeutic approach to overcoming cognitive impairments.
KYNA influences sleep behavior
Only about 5% of dietary tryptophan, which is elevated in serum and brain after prolonged wakefulness and implicated in modulating sleep, is degraded to serotonin and melatonin. The vast majority of tryptophan is metabolized in the kynurenine pathway, which is also responsible for the neosynthesis of KYNA. Yet, the direct relationship between sleep and kynurenine pathway metabolism remains elusive. Our preclinical studies show that brain KYNA levels are elevated in the hippocampus with sleep deprivation, and pharmacological increases in KYNA adversely impacts sleep architecture. Through in vivo electrophysiology, we are investigating how sleep disruption and resulting disruptions in kynurenine pathway metabolism instigate instability in sleep-wake homeostasis and downstream cognitive dysfunction.
Models to study behavioral endophenotypes relevant to neurodevelopmental disorders
Our research investigates pathophysiology of neurodevelopmental disorders in greater depth by examining the neurobiological effects of chronically increased brain KYNA levels during sensitive periods of brain development. To this end, we have developed a model wherein brain KYNA levels are increased prenatally, in line with the neurodevelopmental hypothesis of psychiatric illnesses (Notarangelo and Pocivavsek, 2017). Most recently, we are investigating treatments with a brain-penetrable KYNA synthesis inhibitor to attenuate biochemical abnormalities, memory deficits, and sleep disruptions.